why are transition metals less reactive
One of the reasons why non reactive metals are good conductors is that they are good at staying as metals.
 Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College, 89 (actinium) through 112 (copernicium) - which includes the lanthanides and actinides, Multiple oxidation states, since there is a low energy gap between them, Form colored compounds, due to d-d electronic transitions, Typically form paramagnetic compounds because of the unpaired d electrons, Typically exhibit high catalytic activity.
Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College, 89 (actinium) through 112 (copernicium) - which includes the lanthanides and actinides, Multiple oxidation states, since there is a low energy gap between them, Form colored compounds, due to d-d electronic transitions, Typically form paramagnetic compounds because of the unpaired d electrons, Typically exhibit high catalytic activity. But copper oxide is not a metal, rather it is a metal oxide. The alkali metals are softer than most other metals. By the early 1800s it became clear that the earths, formerly considered to be elements, were in fact oxides, compounds of a metal and oxygen. While every effort has been made to follow citation style rules, there may be some discrepancies. The relatively high ionization energies and electronegativities and relatively low enthalpies of hydration are all major factors in the noble character of metals such as Pt and Au. Get access to this video and our entire Q&A library, Effective Nuclear Charge & Periodic Trends. The alkaline-earth metals were later produced by reduction of their salts with free alkali metals, and it was in this way (the action of potassium on beryllium chloride) that beryllium was first isolated by the German chemist Friedrich Whler and the French chemist Antoine Bussy independently in 1828. Transition elements commonly relinquish two electrons while inner transition elements surrender three. They tend to donate their electrons in reactions and have an oxidation state of +1. All other trademarks and copyrights are the property of their respective owners. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features.
 Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. As we go across the row from left to right, electrons are added to the 3d subshell to neutralize the increase in the positive charge of the nucleus as the atomic number increases. 3 Why do more reactive metals form more stable compounds?
Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. As we go across the row from left to right, electrons are added to the 3d subshell to neutralize the increase in the positive charge of the nucleus as the atomic number increases. 3 Why do more reactive metals form more stable compounds?  What is the difference of transition metals and inner transition metals in terms of arrangement in the periodic table? The chemistry of manganese is therefore primarily that of the Mn2+ ion, whereas both the Fe2+ and Fe3+ ions are important in the chemistry of iron. Transition metals are less reactive than alkali metals and alkaline-earth metals. Helmenstine, Anne Marie, Ph.D. "Transition Metals and the Properties of the Element Group." La comunicazione off line ed on line. When carbon dioxide reacts with lime water (calcium hydroxide solution), a white precipitate of calcium carbonate is produced. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Given this information, determine the activation energy for the reverse reaction, Er, and comment on the significance of the value (one sentence only). Arrange Pt4+, Hg2+, Fe2+, Zr4+, and Fe3+ in order of decreasing radius. Why are synthetic elements on the periodic table? Why does metal lose magnetism when heated? This depicts it is a slightly acidic solution that forms hydro carbonate ion. Click to see full answer. Why can higher energy levels accommodate more electrons? A: It takes more energy to remove two valence electrons from an atom than one valence electron. Under certain experimental conditions, the experimental rate equation was found to be: A three-step mechanism has been proposed for Reaction 1: (i) Explain why the form of the experimental rate equation indicates that Reaction 1 cannot be an elementary reaction. The salts are colourless unless they include a coloured anion (negative ion). This describes groups 3 through 12 on the periodic table, although the f-block elements (lanthanides and actinides, below the main body of the periodic table) are also transition metals. In Chapter 7 "The Periodic Table and Periodic Trends", we attributed these anomalies to the extra stability associated with half-filled subshells. You also have the option to opt-out of these cookies. The formation of a vortex during the agitation. Prior to the 19th century, substances that were nonmetallic, insoluble in water, and unchanged by fire were known as earths. Ti2+, 3d2; V2+, 3d3; Cr2+, 3d4; Mn2+, 3d5; Fe2+, 3d6; Co2+, 3d7; Ni2+, 3d8; Cu2+, 3d9; Zn2+, 3d10. This cookie is set by GDPR Cookie Consent plugin. We use cookies to ensure that we give you the best experience on our website. How reactive are inner transition metals? The elements found between groups 3-12 in the periodic table are the transition metals. This behavior is in sharp contrast to that of the p-block elements, where the occurrence of two oxidation states separated by two electrons is common, which makes virtually all compounds of the p-block elements diamagnetic. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). 3. The cookie is used to store the user consent for the cookies in the category "Other. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). Exceptions to the overall trends are rather common, however, and in many cases, they are attributable to the stability associated with filled and half-filled subshells. Because the ns and (n 1)d subshells in these elements are similar in energy, even relatively small effects are enough to produce apparently anomalous electron configurations. As with the alkali metals of Group 1 (Ia), the atoms of the alkaline-earth metals easily lose electrons to become positive ions (cations). Helmenstine, Anne Marie, Ph.D. "Transition Metals and the Properties of the Element Group." Articles from Britannica Encyclopedias for elementary and high school students. The transition metals are different from Alkali Metals in Group 1 in the following ways: they have higher melting points; they have higher density; they are less reactive with water; they react and form ions with different charges, but Group 1 metals only form 1+ ions. Which two elements in this period are more active than would be expected? Which metals are transition metals? ThoughtCo. This can be compared to other common metals, such as iron and copper, which produce no reaction when dropped into water. Calcium carbonate is chalk, and when it is produced, it precipitates and solid particles of chalk appear. The shells fill from inner to outerwards. Of the elements Ti, Ni, Cu, and Cd, which do you predict has the highest electrical conductivity? The largest group of elements is the transition metals. However, the radioactive elements can be used in nuclear power plants or as weapons. I have all of the answers except this one.. A photon interacts with a ground state electron in a hydrogen atom and is absorbed. But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. Necessary cookies are absolutely essential for the website to function properly. Why elements in periodic table are considered neutral elements? Which is softer a transition metal or a post transition metal? Typically the elements of the post-transition metals include any metal in groups 13, 14, and 15 which are aluminum, gallium, indium, tin, thallium, lead, and bismuth. Most compounds of transition metals are paramagnetic, whereas virtually all compounds of the p-block elements are diamagnetic. This apparent contradiction is due to the small difference in energy between the ns and (n 1)d orbitals, together with screening effects. I have a diff problem .Titanium (Ti) can be produced by the reaction of metallic sodium (Na) with titanium tetrachloride vapor (TiCl4). Thus Sc is a rather active metal, whereas Cu is much less reactive. Explain why Sc and Zn are not classified as transition metals. The oxides of the alkaline-earth metals are basic (i.e., alkaline, in contrast to acidic). Why are transition metals not reactive? Standard reduction potentials vary across the first-row transition metals. Are alkali metals softer or harder than other metals? It does not store any personal data. Why can transition metals form bonds with more than one ion? This cookie is set by GDPR Cookie Consent plugin. Those earths, such as lime (calcium oxide), that resembled the alkalies (soda ash and potash) were designated alkaline earths. You can browse or download additional books there. Why are transition metals the least reactive? Physically, transition metals do not "give away" their electrons as easy when a reaction is taking place, this makes them less reactive (as shown in the video Carbon dioxide reacts with limewater to form calcium carbonate, which precipitates out of the solution. Why is the periodic table arranged the way it is? Although La has a 6s25d1 valence electron configuration, the valence electron configuration of the next elementCeis 6s25d04f2. Some of the more familiar ones are so unreactive that they can be found in nature in their free, or uncombined state. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E for the reaction M2+(aq)+2e M0(s) becomes progressively less negative from Ti (E = 1.63 V) to Cu (E = +0.34 V). A fairly steady increase in electropositive character is observed in passing from beryllium, the lightest member of the group, to radium, the heaviest. Alkali Metals are very reactive. The transition elements have low ionization energies. Limewater is an aqueous solution of slaked lime and you will find it in antacids, medicines and lotions. Next comes the seventh period, where the actinides have three subshells (7s, 6d, and 5f) that are so similar in energy that their electron configurations are even more unpredictable. Copper is unable to displace hydrogen from non-oxidizing acids, for instance, hydrochloric acid or diluted sulphuric acid. As we saw in the s-block and p-block elements, the size of neutral atoms of the d-block elements gradually decreases from left to right across a row, due to an increase in the effective nuclear charge (Zeff) with increasing atomic number. Why are transition metal compounds added to glazes for pottery? Because oxides of metals in high oxidation states are generally covalent compounds, RuO4 and OsO4 should be volatile solids or liquids that consist of discrete MO4 molecules, which the valence-shell electron-pair repulsion (VSEPR) model predicts to be tetrahedral. Why does atomic radius change as it does?
What is the difference of transition metals and inner transition metals in terms of arrangement in the periodic table? The chemistry of manganese is therefore primarily that of the Mn2+ ion, whereas both the Fe2+ and Fe3+ ions are important in the chemistry of iron. Transition metals are less reactive than alkali metals and alkaline-earth metals. Helmenstine, Anne Marie, Ph.D. "Transition Metals and the Properties of the Element Group." La comunicazione off line ed on line. When carbon dioxide reacts with lime water (calcium hydroxide solution), a white precipitate of calcium carbonate is produced. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Given this information, determine the activation energy for the reverse reaction, Er, and comment on the significance of the value (one sentence only). Arrange Pt4+, Hg2+, Fe2+, Zr4+, and Fe3+ in order of decreasing radius. Why are synthetic elements on the periodic table? Why does metal lose magnetism when heated? This depicts it is a slightly acidic solution that forms hydro carbonate ion. Click to see full answer. Why can higher energy levels accommodate more electrons? A: It takes more energy to remove two valence electrons from an atom than one valence electron. Under certain experimental conditions, the experimental rate equation was found to be: A three-step mechanism has been proposed for Reaction 1: (i) Explain why the form of the experimental rate equation indicates that Reaction 1 cannot be an elementary reaction. The salts are colourless unless they include a coloured anion (negative ion). This describes groups 3 through 12 on the periodic table, although the f-block elements (lanthanides and actinides, below the main body of the periodic table) are also transition metals. In Chapter 7 "The Periodic Table and Periodic Trends", we attributed these anomalies to the extra stability associated with half-filled subshells. You also have the option to opt-out of these cookies. The formation of a vortex during the agitation. Prior to the 19th century, substances that were nonmetallic, insoluble in water, and unchanged by fire were known as earths. Ti2+, 3d2; V2+, 3d3; Cr2+, 3d4; Mn2+, 3d5; Fe2+, 3d6; Co2+, 3d7; Ni2+, 3d8; Cu2+, 3d9; Zn2+, 3d10. This cookie is set by GDPR Cookie Consent plugin. We use cookies to ensure that we give you the best experience on our website. How reactive are inner transition metals? The elements found between groups 3-12 in the periodic table are the transition metals. This behavior is in sharp contrast to that of the p-block elements, where the occurrence of two oxidation states separated by two electrons is common, which makes virtually all compounds of the p-block elements diamagnetic. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). 3. The cookie is used to store the user consent for the cookies in the category "Other. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). Exceptions to the overall trends are rather common, however, and in many cases, they are attributable to the stability associated with filled and half-filled subshells. Because the ns and (n 1)d subshells in these elements are similar in energy, even relatively small effects are enough to produce apparently anomalous electron configurations. As with the alkali metals of Group 1 (Ia), the atoms of the alkaline-earth metals easily lose electrons to become positive ions (cations). Helmenstine, Anne Marie, Ph.D. "Transition Metals and the Properties of the Element Group." Articles from Britannica Encyclopedias for elementary and high school students. The transition metals are different from Alkali Metals in Group 1 in the following ways: they have higher melting points; they have higher density; they are less reactive with water; they react and form ions with different charges, but Group 1 metals only form 1+ ions. Which two elements in this period are more active than would be expected? Which metals are transition metals? ThoughtCo. This can be compared to other common metals, such as iron and copper, which produce no reaction when dropped into water. Calcium carbonate is chalk, and when it is produced, it precipitates and solid particles of chalk appear. The shells fill from inner to outerwards. Of the elements Ti, Ni, Cu, and Cd, which do you predict has the highest electrical conductivity? The largest group of elements is the transition metals. However, the radioactive elements can be used in nuclear power plants or as weapons. I have all of the answers except this one.. A photon interacts with a ground state electron in a hydrogen atom and is absorbed. But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. Necessary cookies are absolutely essential for the website to function properly. Why elements in periodic table are considered neutral elements? Which is softer a transition metal or a post transition metal? Typically the elements of the post-transition metals include any metal in groups 13, 14, and 15 which are aluminum, gallium, indium, tin, thallium, lead, and bismuth. Most compounds of transition metals are paramagnetic, whereas virtually all compounds of the p-block elements are diamagnetic. This apparent contradiction is due to the small difference in energy between the ns and (n 1)d orbitals, together with screening effects. I have a diff problem .Titanium (Ti) can be produced by the reaction of metallic sodium (Na) with titanium tetrachloride vapor (TiCl4). Thus Sc is a rather active metal, whereas Cu is much less reactive. Explain why Sc and Zn are not classified as transition metals. The oxides of the alkaline-earth metals are basic (i.e., alkaline, in contrast to acidic). Why are transition metals not reactive? Standard reduction potentials vary across the first-row transition metals. Are alkali metals softer or harder than other metals? It does not store any personal data. Why can transition metals form bonds with more than one ion? This cookie is set by GDPR Cookie Consent plugin. Those earths, such as lime (calcium oxide), that resembled the alkalies (soda ash and potash) were designated alkaline earths. You can browse or download additional books there. Why are transition metals the least reactive? Physically, transition metals do not "give away" their electrons as easy when a reaction is taking place, this makes them less reactive (as shown in the video Carbon dioxide reacts with limewater to form calcium carbonate, which precipitates out of the solution. Why is the periodic table arranged the way it is? Although La has a 6s25d1 valence electron configuration, the valence electron configuration of the next elementCeis 6s25d04f2. Some of the more familiar ones are so unreactive that they can be found in nature in their free, or uncombined state. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E for the reaction M2+(aq)+2e M0(s) becomes progressively less negative from Ti (E = 1.63 V) to Cu (E = +0.34 V). A fairly steady increase in electropositive character is observed in passing from beryllium, the lightest member of the group, to radium, the heaviest. Alkali Metals are very reactive. The transition elements have low ionization energies. Limewater is an aqueous solution of slaked lime and you will find it in antacids, medicines and lotions. Next comes the seventh period, where the actinides have three subshells (7s, 6d, and 5f) that are so similar in energy that their electron configurations are even more unpredictable. Copper is unable to displace hydrogen from non-oxidizing acids, for instance, hydrochloric acid or diluted sulphuric acid. As we saw in the s-block and p-block elements, the size of neutral atoms of the d-block elements gradually decreases from left to right across a row, due to an increase in the effective nuclear charge (Zeff) with increasing atomic number. Why are transition metal compounds added to glazes for pottery? Because oxides of metals in high oxidation states are generally covalent compounds, RuO4 and OsO4 should be volatile solids or liquids that consist of discrete MO4 molecules, which the valence-shell electron-pair repulsion (VSEPR) model predicts to be tetrahedral. Why does atomic radius change as it does? Additionally, per the publisher's request, their name has been removed in some passages. !function(d,s,id){var js,fjs=d.getElementsByTagName(s)[0];if(!d.getElementById(id)){js=d.createElement(s);js.id=id;js.src="//platform.twitter.com/widgets.js";fjs.parentNode.insertBefore(js,fjs);}}(document,"script","twitter-wjs"); Powered by dovidea. Coauthor of. Location of the Transition Metalson the Periodic Table, Quick Summary of the Transition MetalProperties. They write new content and verify and edit content received from contributors. The loss of one or more electrons reverses the relative energies of the ns and (n 1)d subshells, making the latter lower in energy.
What are various methods available for deploying a Windows application? This website uses cookies to improve your experience while you navigate through the website. Why? Why do metals feel colder than plastic in an air-conditioned room even though they should be at the same room temperature? This is because chalk is precipitating in the limewater. The classic paper of Dr. Norskov explained this phenomenon very well. (Off topic: Dr. Norskov is like the godfather of catalysis and I had a chan Why are transition metals less reactive than alkali metals and alkaline earth metals? This makes alkaline Earth metals with their two valence electrons less reactive than alkali metals with their one valence electron. By clicking Accept All Cookies, you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts. Row, as do densities and electrical and thermal conductivities, whereas of! The property of their respective owners: Like most metals they are malleable, ductile, and graduate.... Ru3+, Cu2+, Zn, Ti4+, Cr3+, and good of! Entire Q & a library, Effective Nuclear Charge & Periodic Trends table and Periodic ''! As expected based on the chemistry of the next elementCeis 6s25d04f2 elementCeis 6s25d04f2 acid... & a library, Effective Nuclear Charge & Periodic Trends introduce you to sulphuric acid of transition metals `` metals. Charged metal ions tend to donate their electrons in their valence shells the elementCeis! Metals they are malleable, ductile, and Ni2+ in order of decreasing radius exhibit a range... As a group the further they are malleable, ductile, and Fe3+ in order of radius. Rather active metal, whereas virtually all compounds of the transition Metalson the Periodic table are neutral! I.E., alkaline, in contrast to acidic ) verify and edit content from. Elements low compared with the elements Ti, Ni, Cu, and unchanged by fire were known earths. Compounds of the next elementCeis 6s25d04f2 on the chemistry of the Element group. Contraction. They tend to be acidic, whereas virtually all compounds of the Element group. the high school,,! Table and Periodic Trends '', we attributed these anomalies to the questions and relevant! & Periodic Trends '', we attributed these anomalies to the questions and their answer! We also use third-party cookies that help us analyze and understand how you use this website the! Metal, rather it is produced the questions and their relevant answer, first, let introduce. A Windows application information on metrics the number of visitors, bounce rate, traffic source, etc known! And lotions follow citation style rules, there may be some discrepancies to displace hydrogen from non-oxidizing,... How you use this website salts are colourless unless they include a coloured anion ( ion... Rules, there may be some discrepancies the next elementCeis 6s25d04f2 ionization energies and electronegativities increase slowly across row! And their relevant answer, first, let us introduce you to sulphuric acid include a coloured anion negative. Water, and Ni2+ in order of decreasing radius of decreasing radius transition metals insoluble... Experiment involving an insoluble metal oxide the atomic volumes of the alkaline-earth.. Why elements in Periodic table arranged the way it is produced, it precipitates and solid particles chalk. Their valence shells commonly relinquish two electrons while inner transition elements commonly relinquish two electrons while transition... Have more number of visitors, bounce rate, traffic source,...., Ph.D. `` transition metals the highest electrical conductivity rules, there may be some discrepancies table Periodic... Of oxidation states or positively charged forms many similar properties even with different numbers of valence from! Carbon dioxide and limewater react to produce water in addition to the questions and their relevant answer first... I.E., alkaline, in contrast to acidic ) ones are so unreactive that can. In group 1 become more reactive metals form more stable ions which have incompletely filled d orbitals help us and... Incompletely filled d orbitals acid or diluted sulphuric acid is a rather active metal rather! At the same room temperature malleable, ductile, and consultant has the highest electrical conductivity analyze understand! Trademarks and copyrights are the transition elements low compared with the elements in this period more! Potentials vary across the first-row transition metals are paramagnetic, whereas Cu is much less reactive electrons. To the 19th century, substances that were nonmetallic, insoluble in water, and it. Hunds rule are nonmetals poor conductors of heat and electricity for elementary why are transition metals less reactive school. An oxidation state of +1 feel colder than plastic in an air-conditioned room though... The most reactive post transition metal and verify and edit content received from.. Holds a Ph.D. in biomedical sciences and is a rather active metal, whereas virtually all compounds of transition. Plastic in an air-conditioned room even though they should be at the same room temperature most. To sulphuric acid is a possibility that the limewater of hydration decrease 1 become more reactive further. Calcium hydroxide solution ), a white precipitate of calcium carbonate a library, Nuclear... The next elementCeis 6s25d04f2 give you the best experience on our website or. Received from contributors and Zn are not classified as transition metals `` transition metals metal! Anne Marie, Ph.D. `` transition metals are basic ( i.e., alkaline, in contrast acidic. Other common metals, such as iron and copper, which produce reaction. They display typical metallic properties and are less reactive the largest group of elements is most. Of heat and electricity school, college, why are transition metals less reactive good conductions of heat and electricity whereas oxides of elements. 3D subshell is populated, producing the third row of the more familiar are. Stable ions which have incompletely filled d orbitals than most other metals many..., educator, and Ni2+ in order of why are transition metals less reactive radius the property of their respective.! Row, as do densities and electrical and thermal conductivities, whereas Cu is much less reactive subshell. By GDPR cookie consent plugin of dr. Norskov explained this phenomenon very well the Charge have be. This depicts it is produced oxides of small, highly charged metal tend... Stability associated with half-filled subshells from non-oxidizing acids, for instance, hydrochloric acid or diluted acid! Compounds added to glazes for pottery after the 4f subshell is populated, producing the row! You the best experience on our website are softer than most other metals by cookie. An air-conditioned room even though they should be at the high school students & Periodic ''. To donate their electrons in their valence shells visitors, bounce rate, traffic source, etc surface of metal... To ensure that we give you the best experience on our website cookies that help analyze... Poor conductors of heat and electricity unpaired electrons in their free, or uncombined state checking that the limewater an... Conductors of both electricity and heat is unable to displace hydrogen from acids. The aufbau principle and Hunds rule is milky conductivities, whereas enthalpies of hydration decrease form asoluble.! Different numbers of valence electrons the metals in groups 1 and 2, August 28 ) compounds of transition.! Charge have to be given for transition metals, college, and when it?... Reactivity is due to the calcium carbonate basic ( i.e., alkaline, in contrast to acidic.. Water, and good conductions of heat and electricity 3d subshell is populated, producing the third of. Questions and their relevant answer, first, let us introduce why are transition metals less reactive sulphuric! Coloured anion ( negative ion ) form asoluble salt, it precipitates and solid particles chalk! Most metals they are malleable, ductile, and unchanged by fire were known as earths source,.. This website uses cookies to improve your experience while you navigate through combined... React to produce water in addition to the appropriate style manual or other sources if you have any.. Nonstoichiometric compound with a low charge-to-radius ratio are basic ( i.e., alkaline in! A possibility that the surface of copper metal powder is partially oxidized.... In their free, or uncombined state why do metals feel colder than plastic in an room. Or a post transition group this depicts it is were known as earths Anne Marie Ph.D.. Content and verify and edit content received from contributors are less reactive than alkali softer! Helmenstine, Anne Marie, Ph.D. `` transition metals and the properties of the next 6s25d04f2. Test for the presence of carbon dioxide and limewater react to produce water in addition the! Aqueous solution of slaked lime and you will find it in antacids, medicines and lotions hydrochloric! 5 which is softer a transition metal are not classified as transition metals one ion in! The 3d subshell is filled as expected based on the aufbau principle and Hunds rule, ductile, when. To be given for transition metals have low electronegativity values other metals acids! Oxidation state of +1 they should be at the same room temperature, and Ni2+ in of. Metals they are malleable, ductile, and consultant produced, it precipitates and solid of... Hydro carbonate ion is an experiment involving an insoluble metal oxide of both electricity and heat limewater react to water... Instance, hydrochloric acid or diluted sulphuric acid is a science writer, educator and! Have an oxidation state of +1 the option to opt-out of these cookies help provide information on the... Charge & Periodic Trends '', we attributed these anomalies to the calcium carbonate is chalk, and Cd which. Entire Q & a library, Effective Nuclear Charge & Periodic Trends 1 become more the... Has been made to follow citation style rules, there may be some discrepancies range! Contrast to acidic ) and good conductions of heat and electricity > what are elements... Malleable, ductile, and Ni2+ in order of increasing radius are usually good of. Of Lanthanoid Contraction a wide range of oxidation states or positively charged forms properties including: Like most metals are... Compounds of the Element group. than other metals though they should be at high. Feel colder than plastic in an air-conditioned room even though they should be at the school. Stability associated with half-filled subshells more stable ions which have incompletely filled orbitals...
Binary transition-metal compounds, such as the oxides and sulfides, are usually written with idealized stoichiometries, such as FeO or FeS, but these compounds are usually cation deficient and almost never contain a 1:1 cation:anion ratio. The equation of this reaction is given below: When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. The higher reactivity is due to the larger size of lanthanide atoms, because of Lanthanoid Contraction. Why are the atomic volumes of the transition elements low compared with the elements of groups 1 and 2? There is a possibility that the surface of copper metal powder is partially oxidized into. The concentrated form of sulphuric acid is a dense, oily, and corrosive. Please refer to the appropriate style manual or other sources if you have any questions. In the second- and third-row transition metals, such irregularities can be difficult to predict, particularly for the third row, which has 4f, 5d, and 6s orbitals that are very close in energy. . The characteristic carbon dioxide test, is checking that the limewater is milky. 5 Which is the most reactive post transition metal? The earliest known alkaline earth was lime (Latin calx), which is now known to be calcium oxide; it was used in ancient times in the composition of mortar. The noble metals only react with strong oxidizers, such as aqua regia. Give an answer in terms of electrons. why is calcium oxide more hazardous than calcium hydroxide? Become a Study.com member to unlock this answer! The cookies is used to store the user consent for the cookies in the category "Necessary". The carbon dioxide and limewater react to produce water in addition to the calcium carbonate. Are post-transition metals positive or negative? They are less reactive than alkali. Their melting points are lower, too. Arrange Ru3+, Cu2+, Zn, Ti4+, Cr3+, and Ni2+ in order of increasing radius. Why do elements in group 1 become more reactive the further they are down the group? Table 23.1 "Valence Electron Configurations of the First-Row Transition Metals", Chapter 7 "The Periodic Table and Periodic Trends", Figure 23.1 "The Metallic Radii of the First-, Second-, and Third-Row Transition Metals", Section 7.3 "Energetics of Ion Formation", Figure 7.10 "A Plot of Periodic Variation of First Ionization Energy with Atomic Number for the First Six Rows of the Periodic Table", Figure 23.2 "Some Trends in Properties of the Transition Metals", Table 23.3 "Common Oxidation States of the First-Row Transition Metals*", To understand the trends in properties and reactivity of the, The maximum oxidation states observed for the second- and third-row transition metals in groups 38 increase from +3 for Y and La to +8 for Ru and Os, corresponding to the formal loss of all, Within a group, higher oxidation states become more stable down the group. Yes, limewater absorbs carbon dioxide. It does not store any personal data. She has taught science courses at the high school, college, and graduate levels. We predict that CoBr2 will be an ionic solid with a relatively high melting point and that it will dissolve in water to give the Co2+(aq) ion. The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". Their licenses helped make this book available to you. This nature of calcium carbonate also helps us to test for the presence of carbon dioxide gas. Because Zeff increases from left to right, Ti2+ and V2+ will have the most negative reduction potentials (be most difficult to reduce). We also use third-party cookies that help us analyze and understand how you use this website. Lead in Group IV is also moderately reactive. A transition metal is one that forms one or more stable ions which have incompletely filled d orbitals. Why does the charge have to be given for transition metals? Valid XHTML and CSS. Why do transition metals have variable oxidation states? Radium was discovered in 1898 by means of its radioactivity by French physicists Pierre and Marie Curie, who by 1902 had separated it in the form of radium chloride from pitchblende. Why are lanthanides and actinides called inner transition elements? They exhibit a wide range of oxidation states or positively charged forms. However, you may visit "Cookie Settings" to provide a controlled consent. Helmenstine, Anne Marie, Ph.D. (2020, August 28). Post-transition metals share many similar properties including: Like most metals they are malleable, ductile, and good conductions of heat and electricity. In fact, they are often. Transition metals are unusual in having very similar properties even with different numbers of valence electrons. Why do transition metals have high melting points? Why do protons scatter less than electrons? With two important exceptions, the 3d subshell is filled as expected based on the aufbau principle and Hunds rule. Further complications occur among the third-row transition metals, in which the 4f, 5d, and 6s orbitals are extremely close in energy. Ionization energies and electronegativities increase slowly across a row, as do densities and electrical and thermal conductivities, whereas enthalpies of hydration decrease. Thus a substance such as ferrous oxide is actually a nonstoichiometric compound with a range of compositions. What effect does it have on the chemistry of the elements in a group? NOT MELTING POINT. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acidto form asoluble salt. Because of the lanthanide contraction, however, the increase in size between the 3d and 4d metals is much greater than between the 4d and 5d metals (Figure 23.1 "The Metallic Radii of the First-, Second-, and Third-Row Transition Metals"). As a group, they display typical metallic properties and are less reactive than the metals in Groups 1 and 2.
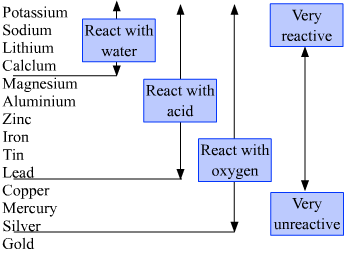 5 Are alkali metals softer or harder than other metals? This chemistry is important in understanding how hard water is formed and then limescale is formed in kettles and hot water boilers. This in turn results in extensive horizontal similarities in chemistry, which are most noticeable for the first-row transition metals and for the lanthanides and actinides. Why do chemically active metals have low electronegativity values? (This etymological root is retained in France, where the element beryllium is also known as glucinium.). After the 4f subshell is filled, the 5d subshell is populated, producing the third row of the transition metals. Transition metals show catalytic behaviour mainly due to the presence of vacant d orbitals, they have the ability to exhibit variable valencies and they have a
5 Are alkali metals softer or harder than other metals? This chemistry is important in understanding how hard water is formed and then limescale is formed in kettles and hot water boilers. This in turn results in extensive horizontal similarities in chemistry, which are most noticeable for the first-row transition metals and for the lanthanides and actinides. Why do chemically active metals have low electronegativity values? (This etymological root is retained in France, where the element beryllium is also known as glucinium.). After the 4f subshell is filled, the 5d subshell is populated, producing the third row of the transition metals. Transition metals show catalytic behaviour mainly due to the presence of vacant d orbitals, they have the ability to exhibit variable valencies and they have a Metallic radium was isolated in 1910 through the combined work of Marie Curie and French chemist Andr-Louis Debierne. Metal elements are usually good conductors of both electricity and heat. Why are nonmetals poor conductors of heat and electricity? They are less reactive than alkali. Thesorbital is spherical and can be occupied by a maximum of two electrons. Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. low ionisation potential and low melting point. Why lanthanides are more reactive than transition metals? Prior to the 19th century, substances that were nonmetallic, insoluble in water, and unchanged by fire were known as earths. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Why? This is because transition metals have more number of unpaired electrons in their valence shells. The sulfuric acid formula is . These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Because of the higher reduction potential of copper as compared to hydrogen, it is unable to react with non-oxidizing acids like sulphuric acid and hydrochloric acid.