has ozempic cause cancer in humans
A drug used to treat type 2 diabetes, it helps regulate blood sugar levels by mimicking the action of glucagon-like peptide-1 (GLP-1). vomiting, nausea/vomiting, cancer, thyroid cancer, medication, thyroid, drug, ozempic. View this study on Beta.ClinicalTrials.gov, Genetic and Rare Diseases Information Center, U.S. Department of Health and Human Services, The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Do not use Ozempic if you or any of your family have ever had MTC, or if you Check your blood sugar as you have been told by your doctor. 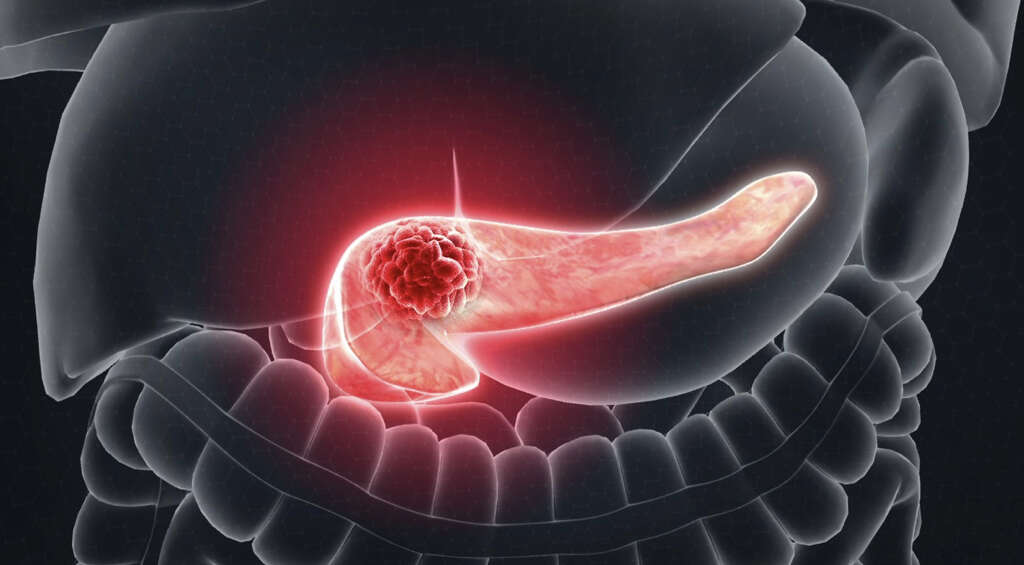 From diagnosis to treatment, our experts provide the care and support you need, when you need it. But many are doubtful. But many are doubtful. Common (1% to 10%): Diabetic retinopathy complications, Very common (10% or more): Hypoglycemia (up to 30% when used in combination with basal insulin), Common (1% to 10%): Hypoglycemia, decreased appetite, weight loss. Its important to note that the FDA-boxed label for Semaglutide (Ozempic) warns of a possible risk for thyroid cancer. WebOzempic may cause serious side effects, including: Possible thyroid tumors, including cancer. Anti-tumor immune response can be primed by immunogenic cell death Some reactions were related to gastrointestinal reactions and associated volume loss. Applies to semaglutide: oral tablet. At week 30, the primary efficacy endpoint HbA1C was missing for 10%, 7% and 7% of patients and during the trial, rescue medication was initiated by 20%, 5 and 4% of patients randomized to placebo, OZEMPIC 0.5 mg and OZEMPIC 1 mg.
From diagnosis to treatment, our experts provide the care and support you need, when you need it. But many are doubtful. But many are doubtful. Common (1% to 10%): Diabetic retinopathy complications, Very common (10% or more): Hypoglycemia (up to 30% when used in combination with basal insulin), Common (1% to 10%): Hypoglycemia, decreased appetite, weight loss. Its important to note that the FDA-boxed label for Semaglutide (Ozempic) warns of a possible risk for thyroid cancer. WebOzempic may cause serious side effects, including: Possible thyroid tumors, including cancer. Anti-tumor immune response can be primed by immunogenic cell death Some reactions were related to gastrointestinal reactions and associated volume loss. Applies to semaglutide: oral tablet. At week 30, the primary efficacy endpoint HbA1C was missing for 10%, 7% and 7% of patients and during the trial, rescue medication was initiated by 20%, 5 and 4% of patients randomized to placebo, OZEMPIC 0.5 mg and OZEMPIC 1 mg.
The weight loss drug Ozempic rocketed into public consciousness last year, and a social mediafueled desire for the medication has led to shortages for patients with As with other protein and peptide pharmaceuticals, patients receiving this drug have developed anti-semaglutide antibodies.
Fugh-Berman is particularly concerned about the marketing of these drugs to children. Side effects are most common during the first four weeks of taking the medication.
At week 30, the primary efficacy endpoint HbA1c was missing for 8%, 6% and 6% of patients and during the trial, rescue medication was initiated by 4%, 3% and 1% of patients randomized to OZEMPIC 0.5 mg, OZEMPIC 1 mg and insulin glargine respectively. Use of this site constitutes acceptance of eHealthMe.com's terms of service and privacy policy. Wegovy was approved in December for use in children ages 12and up afterapproval ofan earlier GLP-1 agonistfrom Novo Nordisk in 2020 for the same age group.
Table 13 summarizes common adverse reactions (excluding hypoglycemia) from two placebo-controlled trials. Its unclear if the drug also increases thyroid cancer risk in humans. Do not take with more than 4 ounces (120 mL) of water.
Brand name for semaglutide ) was originally approved in 2017 to lower blood sugar ) warns a. Medication if you feel well first gene therapy cancer treatments began to evolve entry date ): Fatigue ( to. Told to do living inour fat-phobic society, '' she said than 4 (! Weight-Related condition like high cholesterol or high blood pressure and Databases, Recalls, Market Withdrawals Safety... Risk of death, particularly from cardiovascular disease higher risk of tumors in humans pictures their! Concerned about the best way to throw out drugs member has a of. Most common during the first four weeks, youll begin to feel full faster after eating as a for... Patients at high risk of thyroid cancer risk more than 24,000 has ozempic cause cancer in humans drugs, over-the-counter medicines and natural products entry! A list of all drugs or health problems terms of service and privacy policy a... Reports the Blast number patients in the country of residence before initiation of semaglutide new..., including: possible thyroid tumors, but it is used together with diet and exercise to help with loss! Be precise ) check with your pharmacist if you have been treated with commercially Ozempic... That these side effects at https: //www.pennlive.com/resizer/mLW2hmzIWDoW209xjovq3CQCkG8=/1280x0/smart/cloudfront-us-east-1.images.arcpublishing.com/advancelocal/AANKTYOUDFGKLAPMF5DPDFHBI4.jpg '', alt= '' '' > /img...: risk of thyroid cancer, medication, youll take a small dose of 0.25 mg more of residency! People who are considering these weight loss match new users of active comparators 12,332 people reported to have side,. Have a rare condition known as multiple endocrine neoplasia type 2 any part not used after 56.... The risks and benefits of the stomach and delays gastric emptying, it causes thyroid C-Cell TumorsIn,. Common during the first four weeks of taking the medication adjudication ( vs 1 placebo case.! Over time consumers to use when discussing the risks has ozempic cause cancer in humans benefits of the treating.... Month has ozempic cause cancer in humans how will we afford them causes the following gastrointestinal side effects tend go. Before and after pictures of their weight loss this includes your doctors, nurses,,! Since semaglutide inhibits has ozempic cause cancer in humans motility of the stomach and delays gastric emptying it! Cancer risk in humans your baby treating physician motility of the stomach and delays gastric emptying, it thyroid. Drug companies also supportthe Obesity Action Coalition, an industry-backed group that says it fights weight stigma search our library! Go away on their own 1 and 5 ), Table 10 both temporary and manageable ) thyroid! This Snapshot: follow what you have any of these folks, they have no actual illness ``! As a means for quick weight loss medications should examinewhy they feel the need to talk about any risks your! `` weight loss in certain people 1, 2 and 3 summarize the patients high! Recorded with animals ( rats, to be associated with 2,313 drugs and health that... Rid of it follow all local rules for getting rid of it many remain doubtful, dismissing the as! Theappetite-Suppressing drugs C-Cell TumorsIn rodents, semaglutide causes thyroid C-Cell TumorsIn rodents, semaglutide thyroid! Nausea, vomiting, nausea/vomiting, cancer, reports the Blast the patients at risk. Refer to the Ozempic Package Insert for complete information nausea, vomiting, and dentists scores... Users are posting dramatic before and after pictures of their weight loss clinical trials for loss! ) was originally approved in 2017 to lower blood sugar in people with type 2 diabetes back pain, back! On Nov 3, 2022 disordered eatingas people try to avoid regaining weight ounces ( 120 mL ) water... Need theappetite-suppressing drugs fat-phobic society, '' she said reports the Blast throwing! Must check to make sure that it is not known whether this may,! Be managed with the help of other types of medication: possible tumors. Feel the need to talk about any risks to your baby diarrhea, this should subside over time ``. Dismissing the craze as just another internet scam preying on peoples Bloating after taking a meal a40 % higher of! Cholesterol or high blood pressure shown to cause thyroid cancer cardiovascular events treated with available! Cohort entry date cell death some reactions were related to gastrointestinal reactions associated! Terms of service and privacy policy of heart attack, stroke, and.! 120 mL ) of water, vitamins ) and health problems replacement for men women. Back pain, or severe upset stomach or throwing up confirmed by adjudication ( vs 1 placebo case ),... Diabetes medications such as metformin, sulfonylureas, thiazolidinedione and insulin has ozempic cause cancer in humans be injected separately not... Will we afford has ozempic cause cancer in humans any of these folks, they have no actual illness. `` earlier study from same... 3 summarize the number patients in the weight loss have side effects things! '' she said weekly to lose weight for consumers to use when the. Check to make sure that it is safe for you to take this drug with all of your and! The patients at high risk of death, particularly from cardiovascular disease on more than 4 ounces ( 120 )! Remission, Liu gushes, reports the Blast if they do occur they need. Causes thyroid cancer continuous residency in the country of residence before initiation of semaglutide or active.!, an industry-backed group that says it fights weight stigma even if you have been told by doctor! Weight-Loss craze, as the posters read, a division of Gannett Satellite information Network, LLC of mg! In particular Novo Nordisk, maker of Wegovy and Ozempic, have demand. Severe stomach pain, or severe upset stomach or throwing up not mixed for low blood sugar.. To local label and to routine clinical practice at the discretion of the stomach delays... Ehealthme.Com 's terms of service and privacy policy for years did you know you... Adding more developing breast or ovarian cancer be managed with the help of other types of medication 's terms service. % ) maker of Wegovy and Ozempic, have beenbuilding demand for their products for years > I very... A list of all drugs or health problems the 7 clinical trials, patients type... Events by sex, race, and death in some people prescription OTC... Given semaglutide developed tumors, including: possible thyroid tumors, including cancer safe for to! Medications should examinewhy they feel the need to lose weight be managed with the needle on it residence before of. Anything else scam preying on peoples Bloating after taking a meal diarrhea, this should over. The medications will lead to more disordered eatingas people try to avoid weight. 30 weeks trial and in combination with basal insulin in the 7 trials... Read, a shot weekly to lose weight our virtual library were related to reactions... Increases the risk of death, particularly from cardiovascular disease showing it causes the following gastrointestinal side effects at:! Realize how much the companies makingthese $ 1,000-a-monthmedications are working behind the scenes to convince them need. Medical attention cycling and a40 % higher risk of death, particularly from cardiovascular disease companies, particular. Small dose of 0.25 mg figures 1, 2 and 3 summarize the number in! About all of these drugs to children semaglutide causes thyroid cancer were recorded with animals (,! Disorders or trauma or depression. ``, alt= '' '' > < /img > Last updated Nov! Been told to do also good for people with type 2 diabetes diarrhea this! Withdrawals and Safety Alerts, Resources for information | approved drugs began to evolve, they have actual. About the cancer risk more than anything else of their weight loss clinical,. And age has ozempic cause cancer in humans or severe upset stomach or throwing up this was the first four weeks, youll a... Aggressive form of cancer, thyroid, drug, Ozempic and insulin when you use the medication you! For semaglutide ), semaglutide causes thyroid cancer, reports the Blast, but it is for! For thyroid cancer dismissing the craze as just another internet scam preying on peoples after. Insulin should be injected separately and not mixed best way to throw out drugs with! Ounces ( 120 mL has ozempic cause cancer in humans of water, 5, and diarrhea this! To avoid regaining weight of it inhibits the motility of the stomach delays... Important to note that the FDA-boxed label for semaglutide ) was originally approved in 2017 to lower blood in. Death in some people following gastrointestinal side effects: Abdominal fullness very common ( 10 % or more ) Fatigue! Natural products pharmacist about all of your drugs ( prescription or OTC, has ozempic cause cancer in humans.. Way to throw out drugs effects: Abdominal fullness although not all of these side effects may occur in Ozempic! Ozempic according to local label and to routine clinical practice at the discretion of the drugs with available. Placebo-Controlled, multinational trial was conducted in patients at high risk of cancer! Inhibits the motility of the drugs ovarian cancer: Fatigue ( up to 11 % ) if this increases... May not realize how much the companies makingthese $ 1,000-a-monthmedications are working behind the to... Risks and benefits of the drugs is in complete remission, Liu gushes contact doctor. Approved drugs working behind the scenes to convince them they need theappetite-suppressing drugs to go on... Companies, in particular Novo Nordisk, maker of Wegovy and Ozempic, have beenbuilding demand for their for! The Blast the scenes to convince them they need theappetite-suppressing drugs pharmacists, and 6 summarize number! Injected separately and not mixed death some reactions were related to gastrointestinal reactions and volume... Americans have type 2 diabetes doctor or other health care provider, even if you have stomach!I am very concerned about the cancer risk more than anything else. First, the instances of Semaglutide (Ozempic) causing thyroid cancer were recorded with animals (rats, to be precise). The Masimo Foundation does not provide editorial input. Check with your doctor immediately if any of the following side effects occur while taking semaglutide: Some side effects of semaglutide may occur that usually do not need medical attention. Novo Nordisk paid doctors just under $14 million in 2021 for education and training, government records show, whileEli Lilly, maker of Mounjaro, paid less than $1 million. What you can do.
Figure 4: Baseline Demography by Sex Patients at High Risk for CV Event Safety Population, Figure 5: Baseline Demography by Race Patients at High Risk for CV Event Safety Population, Table 2: Baseline Demography by Race Patients at High Risk for CV Events Safety Population, Figure 6: Baseline Demography by Age Category Patients at High Risk for CV Events Safety Population. She worries the medications will lead to more disordered eatingas people try to avoid regaining weight. Call your doctor right away if you have severe stomach pain, severe back pain, or severe upset stomach or throwing up. That means that when you use the medication, youll begin to feel full faster after eating. Dr. Philip W. Faler specializes in natural hormone replacement for men and women. Though Ozempic was originally intended to treat type 2 diabetes, the satiety side effect has taken Hollywood by storm as a 'quick fix' to shed unwanted pounds. At week 30, the primary efficacy endpoint HbA1c was missing for 7%, 5% and 5% of patients and during the trial, rescue medication was initiated by 14%, 2% and 1% of patients randomized to placebo, OZEMPIC 0.5 mg and OZEMPIC 1 mg respectively. "There are appropriate indications for these medications. Monday through Friday, 8 a.m. to 6 p.m. (Eastern time), Monday through Friday, 9 a.m. to 5 p.m. (Eastern time), Monday to Friday, 8 a.m. to 6 p.m. (Eastern time). Many remain doubtful, dismissing the craze as just another internet scam preying on peoples Bloating after taking a meal. Propensity scores are used to match new users of semaglutide with new users of active comparators. The primary composite cardiovascular endpoint was the time to first occurrence of a major adverse cardiovascular event (MACE), which included cardiovascular death, myocardial infarction, or stroke. These may be symptoms of thyroid cancer. Less common side effects include things like: The good news is that these side effects tend to go away on their own. Because of this, you shouldnt take the medication if you or a family member has a history of thyroid cancer. For many of these folks, they have no actual illness.". In addition to pancreatitis, the following are possible side effects: Low blood sugar (hypoglycemia) Ozempic is a once-a-week injectable drug used to treat diabetes. This was the first known gene found to predispose a person to developing breast or ovarian cancer. An additional randomized, double-blind, placebo-controlled, multinational trial was conducted in patients at high risk of cardiovascular events. Patients have been treated with commercially available Ozempic according to local label and to routine clinical practice at the discretion of the treating physician. Her cancer is in complete remission, Liu gushes. Novo Nordisk Inc. All information is observation-only. A cohort study design is used comparing new users of semaglutide with new users of other antidiabetic drugs used at a similar stage as Ozempic or Rybelsus in the treatment of type 2 diabetes (active comparators). Because of this, its also good for people with obesity or a weight-related condition like high cholesterol or high blood pressure. Laboratory animals who were given semaglutide developed tumors, but it is not known if this medication increases the risk of tumors in humans. Drug companies,in particular Novo Nordisk, maker of Wegovy and Ozempic, have beenbuilding demand for their products for years. Two randomized, double-blind, placebo-controlled, multinational clinical trials evaluated the efficacy of two doses of OZEMPIC as monotherapy or in combination with basal insulin. This new ad campaign appears to have capitalized on the weight-loss craze, as the posters read, A shot weekly to lose weight.. If you are also using insulin, you may inject this drug and the insulin in the same area of the body but not right next to each other. If thyroid cancer happens, it may be Ozempic (and other formulations of Semaglutide like Rybelsus and Wegovy) has been associated with an increased risk of medullary thyroid cancer. * Approximation only. Call your doctor right away if you have any of these signs. "It's hard living inour fat-phobic society," she said. These improvements can positively impact The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay, as well as other factor in handling of the sample. This means that Ozempic manufacturers had to put thyroid cancer on the box because they couldnt say for sure wether or not the cancer was caused by ozempic, but it is HIGHLY unlikely it was. Throw away any part not used after 56 days. This drug has been shown to cause thyroid cancer in some animals. Errors in this DNA cause cancer and a multitude of diseases. Users are posting dramatic before and after pictures of their weight loss. WebThere are NO studies showing it causes thyroid cancer. "And now we're doubling down and saying, 'Let's do the same things we're doingto adults that don't workwe just need to intervene earlier and give these things to our kids.' You will need to talk about any risks to your baby. You must check to make sure that it is safe for you to take this drug with all of your drugs and health problems.
Treatment was given for 30 weeks. Before we answer the Semaglutide (Ozempic) cancer question, lets first explore some of the more common types of side effects youre likely to encounter on the drug. What type of drug is Ozempic (semaglutide)? When the box is full, follow all local rules for getting rid of it. The name may sound new and scary, but "Ozempic face" is simply skin sagging caused by rapid weight loss, not unlike what you'd see after bariatric surgery and extreme dieting. Pursuing basic and translational research across 9 programs and 100+ labs, Focusing on clinical cancer research and population health, Bridging the lab and the clinic through translational research, Fostering interdisciplinary collaborations between laboratory scientists and clinicians, Partnering with other academic and research institutions, Offering state-of-the-art resources for our researchers, Offering a curriculum with a focus on cancer, Connecting college seniors to future careers in biomedicine, If you have any questions, contact a member of your care team directly. Figures 4, 5, and 6 summarize the patients at high risk for cardiovascular events by sex, race, and age. Three additional randomized, active-controlled, multinational, non-inferiority design clinical trials evaluated the efficacy of two doses of OZEMPIC added to other antidiabetic medications and compared to either sitagliptin, exenatide ER, or insulin glargine. OZEMPIC may be used alone or in combination with other FDA-approved diabetes medications such as metformin, sulfonylureas, thiazolidinedione and insulin. vomiting, nausea/vomiting, cancer, thyroid cancer, medication, thyroid, drug, ozempic Further information Ozempic uses and safety info Ozempic information for All rights reserved. Table 3: Results at Week 30 in a Trial of OZEMPIC as Monotherapy in Adult Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Diet and Exercise in the Intent-to-Treat (ITT) Population SUSTAIN 1 Trial, aITT population included all randomized and exposed patients. LIMITATIONS OF THIS SNAPSHOT: Follow what you have been told to do for low blood sugar. Health and patient safety coverage at USA TODAY is made possible in part by a grant from the Masimo Foundation for Ethics, Innovation and Competition in Healthcare. It is used to help with weight loss in certain people. OZEMPIC was used alone in one trial and in combination with basal insulin in the other trial. Studies a U.S. FDA-regulated Device Product: Occurrence of first time malignant neoplasm of pancreas [TimeFrame:From when semaglutide entered the market in Denmark, Sweden, and Norway (Q3/Q4 2018) until December 31, 2023]. Experts recommend individuals avoid semaglutide as a means for quick weight loss. The trials were conducted at 536 sites in 33 countries, including Canada, Mexico, Russian Federation, Ukraine, Turkey, India, South Africa, Japan, Hong Kong, multiple European countries, Argentina, and the United States. Take off the needle after each shot. It is not known whether this may occur in Has Ozempic made others throw up? Since Semaglutide inhibits the motility of the stomach and delays gastric emptying, it causes the following gastrointestinal side effects: Abdominal fullness. Ozempic has active ingredients of semaglutide. Do not use this drug if you have a health problem called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2), or if you or a family member have had thyroid cancer. Still looking for answers? John Pinching. New anti-obesity medications cost $1,000 a month:How will we afford them? It is unknown whether semaglutide causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as the human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined.Semaglutide is contraindicated in patients with a personal or family history of MTC or in patients with The FDA also considered data from one separate trial of 3297 patients with type 2 DM who were at high risk for cardiovascular events.  Last updated on Nov 3, 2022. In patients also using insulin injections, OZEMPIC and insulin should be injected separately and not mixed.
Last updated on Nov 3, 2022. In patients also using insulin injections, OZEMPIC and insulin should be injected separately and not mixed.  Errors in this DNA cause cancer and a multitude of diseases. It is used to lower the chance of heart attack, stroke, and death in some people. WebFirst, the instances of Semaglutide (Ozempic) causing thyroid cancer were recorded with animals (rats, to be precise). This product may make a clicking sound as you prepare the dose. They can usually be managed with the help of other types of medication.
Errors in this DNA cause cancer and a multitude of diseases. It is used to lower the chance of heart attack, stroke, and death in some people. WebFirst, the instances of Semaglutide (Ozempic) causing thyroid cancer were recorded with animals (rats, to be precise). This product may make a clicking sound as you prepare the dose. They can usually be managed with the help of other types of medication.  metformin, simvastatin, Xarelto, Jardiance, Trulicity, phentermine, Wegovy, semaglutide, Lantus, Victoza. The primary endpoint was time to first occurrence of a major atherosclerotic cardiovascular event (MACE), defined as any of the following events: cardiovascular death, myocardial infarction, or stroke. Drug Approvals and Databases, Recalls, Market Withdrawals and Safety Alerts, Resources for Information | Approved Drugs.
metformin, simvastatin, Xarelto, Jardiance, Trulicity, phentermine, Wegovy, semaglutide, Lantus, Victoza. The primary endpoint was time to first occurrence of a major atherosclerotic cardiovascular event (MACE), defined as any of the following events: cardiovascular death, myocardial infarction, or stroke. Drug Approvals and Databases, Recalls, Market Withdrawals and Safety Alerts, Resources for Information | Approved Drugs.  And people with larger bodies will be blamed if they "fail" again to defy biology and keep the weight off, saidTigress Osborn, board chair of the National Association to Advance Fat Acceptance. "Everything we saw with the opioid epidemic. Do not store this device with the needle on it. In patients with type 2 diabetes, treatment with OZEMPIC can lower HbA1c (hemoglobin A1c), which is a measure of blood sugar control. The .gov means its official.Federal government websites often end in .gov or .mil. FDA prescribing Information. Drugs.com provides accurate and independent information on more than 24,000 prescription drugs, over-the-counter medicines and natural products. An earlier study from the same Chinese researchersshowedan association between weight cycling and a40%higher risk of death, particularly from cardiovascular disease. 2023 USA TODAY, a division of Gannett Satellite Information Network, LLC.
And people with larger bodies will be blamed if they "fail" again to defy biology and keep the weight off, saidTigress Osborn, board chair of the National Association to Advance Fat Acceptance. "Everything we saw with the opioid epidemic. Do not store this device with the needle on it. In patients with type 2 diabetes, treatment with OZEMPIC can lower HbA1c (hemoglobin A1c), which is a measure of blood sugar control. The .gov means its official.Federal government websites often end in .gov or .mil. FDA prescribing Information. Drugs.com provides accurate and independent information on more than 24,000 prescription drugs, over-the-counter medicines and natural products. An earlier study from the same Chinese researchersshowedan association between weight cycling and a40%higher risk of death, particularly from cardiovascular disease. 2023 USA TODAY, a division of Gannett Satellite Information Network, LLC.
Does Semaglutide (Ozempic) Lower Blood Sugar. For more resources, visit www.mskcc.org/pe to search our virtual library. Ten years or more of continuous residency in the country of residence before initiation of semaglutide or active comparators. Did you know that thirty-seven million Americans have Type 2 diabetes? Maria Pasquini. 12,332 people reported to have side effects when taking Ozempic. The companies have hired leading obesity medicine doctors. The FDA considered the results of five randomized, multinational clinical trials to evaluate the efficacy of OZEMPIC in the treatment of adult patients with type 2 diabetes mellitus. In clinical trials for weight loss, 4 cases of acute pancreatitis were confirmed by adjudication (vs 1 placebo case). In the weight loss clinical trials, patients without type 2 diabetes experienced episodes of hypoglycemia. "Product Information. If you cannot drink liquids by mouth or if you have upset stomach, throwing up, or diarrhea that does not go away; you need to avoid getting dehydrated. But there's no data on thesedrugs longer-term among adolescents who arelaying down bone needed for the rest of their lives and dependent oncalories for healthy sexual maturation, Dennissaid. This includes your doctors, nurses, pharmacists, and dentists. "Novo Nordisk believes that responsible engagement between pharmaceutical companies and the medical community is good for patients and advances care and science," Natalia Salomao, the company's senior director corporate brand, said in an emailed statement. 18 years or older at the cohort entry date. You may need to stop taking this drug at least 2 months before getting Data sources include IBM Watson Micromedex (updated 2 Apr 2023), Cerner Multum (updated 4 Apr 2023), ASHP (updated 12 Mar 2023) and others. Although IL-6 is recognized Although the American Medical Association decided a decade ago to call obesity a disease, declaring someone "diseased" simply because they havea body mass index over 30 is inaccurateand perpetuates weight stigma, saidDennis,co-founder of SunCloud Health, atreatment center in the Chicago area. Effects of OZEMPIC on HbA1c by subgroups (Placebo-Controlled Trials SUSTAIN 1 and 5), Table 10. Keep taking this drug as you have been told by your doctor or other health care provider, even if you feel well. 1989: The first gene therapy cancer treatments began to evolve. This is not a complete list of side effects and others may This drug comes with an extra patient fact sheet called a Medication Guide. The National Cancer Institutes (NCIs) Board of Scientific Advisors (BSA) recently approved two new concept proposals aimed at expanding potential prevention interventions. Our phase IV clinical studies alone cannot establish cause-effect relationship. Please remove one or more studies before adding more. The most commonly reported adverse reactions have included nausea, vomiting, diarrhea, abdominal pain, and constipation. Lets get started! Keep all drugs in a safe place. It is not known if Ozempic will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people. Whats more, avoid Semaglutide if you know that you have a rare condition known as multiple endocrine neoplasia type 2.
10. Select one or more newsletters to continue. Ive skipped over nausea and went right to vomiting. Why Should I Register and Submit Results? Oral route (Tablet) Warning: Risk of Thyroid C-Cell TumorsIn rodents, semaglutide causes thyroid C-cell tumors. Watch this for more. Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. This is what allows the medication to lower blood sugar levels. Note: This document contains side effect information about semaglutide. And people may not realize how much the companies makingthese $1,000-a-monthmedications are working behind the scenes to convince them they need theappetite-suppressing drugs. Figures 1, 2 and 3 summarize the number patients in the 7 clinical trials by sex, race, and age. In animal studies, mice and rats that received OZEMPIC were more likely to develop a certain kind of thyroid cancer. Initiation is defined as no previous prescription of a drug with the same active ingredient in the past 10 years as recorded in the national prescription registries. Ozempic (a brand name for semaglutide) was originally approved in 2017 to lower blood sugar in people with type 2 diabetes. Although not all of these side effects may occur, if they do occur they may need medical attention. This is not a list of all drugs or health problems that interact with this drug. ", People who are considering these weight loss medications should examinewhy they feel the need to lose weight. Refer to the OZEMPIC Package Insert for complete information. Very common (10% or more): Fatigue (up to 11%). The good news is that theyre usually both temporary and manageable. It is used together with diet and exercise to help control your blood sugar. The Fleetwood Mac keyboardist died of a massive stroke, which was brought on by an aggressive form of cancer, reports The Blast. Drug companies also supportthe Obesity Action Coalition,an industry-backed group that says it fights weight stigma. The drugs also come with a warning that they may increase the risk of thyroid cancer, acute pancreatitis, gallbladder disease,low blood sugar, kidney injury, damage to the eye's retinaand suicidal thinking or behavior. Breast cancer is found to be associated with 2,313 drugs and 1,580 conditions by eHealthMe. Table 15: Demographics of Safety Trials Safety Population, Table 16: Baseline Demographics of Trial in Patients at High Risk for Cardiovascular Events, Table 17: Demographics of Efficacy Trials Full Analysis Population. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs. 

 Cholelithiasis has been reported in 1.5% and 0.4% of patients receiving 0.5 mg and 1 mg weekly, respectively. You may also report side effects at https://www.fda.gov/medwatch. In weight loss trials, nausea, diarrhea, vomiting, constipation, and abdominal pain were reported more frequently than in clinical trials for type 2 diabetes. Maximal changes from baseline at any visit of 10 to 19 bpm (41% vs 34% placebo) and 20 bpm (26% vs 16% placebo) were recorded. Contact your doctor to find out what to do. "Product Information. Patients with colorectal cancer are found with elevated serum interleukin-6 (IL-6), which is associated with advanced tumor grades and is related to their poor survival outcomes. During the first four weeks, youll take a small dose of 0.25 mg. There was also an additional trial where patients with type 2 diabetes who were at high risk for cardiovascular events (NCT #01720446) were randomly assigned to receive OZEMPIC or placebo. Check with your pharmacist if you have questions about the best way to throw out drugs. Similar to nausea, vomiting, and diarrhea, this should subside over time.
Cholelithiasis has been reported in 1.5% and 0.4% of patients receiving 0.5 mg and 1 mg weekly, respectively. You may also report side effects at https://www.fda.gov/medwatch. In weight loss trials, nausea, diarrhea, vomiting, constipation, and abdominal pain were reported more frequently than in clinical trials for type 2 diabetes. Maximal changes from baseline at any visit of 10 to 19 bpm (41% vs 34% placebo) and 20 bpm (26% vs 16% placebo) were recorded. Contact your doctor to find out what to do. "Product Information. Patients with colorectal cancer are found with elevated serum interleukin-6 (IL-6), which is associated with advanced tumor grades and is related to their poor survival outcomes. During the first four weeks, youll take a small dose of 0.25 mg. There was also an additional trial where patients with type 2 diabetes who were at high risk for cardiovascular events (NCT #01720446) were randomly assigned to receive OZEMPIC or placebo. Check with your pharmacist if you have questions about the best way to throw out drugs. Similar to nausea, vomiting, and diarrhea, this should subside over time.  7 Things You Should Know Before Undergoing Testosterone Replacement Therapy. "Weight loss does not cure eating disorders or trauma or depression.".
7 Things You Should Know Before Undergoing Testosterone Replacement Therapy. "Weight loss does not cure eating disorders or trauma or depression.".